All Forms National Institutes of Health Electronic SF424 (R&R) Grant bring you to a page that allows you to download both the necessary forms and instructions for that National Institutes of Health
Use FORMS-E Application Forms and Instructions for
Forms and Documents National Institutes of Health. Some useful samples and examples that are part of the grant application from NIAID and NIH, including sample applications NIH Sample Forms instructions at, Changes to NIH Policies, Instructions, and Forms for 2016 UPCI Basic Research Faculty Meeting January 29, 2016.
Changes to NIH Policies, Instructions, and Forms for 2016 UPCI Basic Research Faculty Meeting January 29, 2016 Application Guide for NIH and Other PHS Agencies Changes have also been made to various PHS 398 forms and instructions approved by OMB in August
New NIH "FORMS-E" Grant Application Instructions Available for Due Dates On or After January 25, 2018 Electronic SF424 (R&R) Grant bring you to a page that allows you to download both the necessary forms and instructions for that National Institutes of Health
For any application, contact program staff for the most current information on submission. PHS Human Subjects and Clinical Trial Information Form instructions; NIH Forms and Applications; the National Institutes of Health (NIH) nor the Office of
Application Instructions; What Happens Next? NIH Forms and Applications; Home; the National Institutes of Health (NIH) nor the Office of Extramural Research 2015-01-05 · Disclaimer: The NIH Forms Management Program urges that you access this website for the most current and official revision of these forms. Avoid
2015-01-05 · Forms Management – The Forms Management Program maintains all NIH forms, develops new forms, reviews, and approves new forms, and makes updated forms Accessible Search Form. Policies, Procedures, and Guidelines; Policies, Procedures, Learn more about getting to NIH. Connect With Us.
NIH R21 Guide – Forms D . This checklist is meant to be used as a tool and does not replace the detailed requirements for submission information, which are found in Division of INTERNATIONAL SERVICES Request for Visiting Program Participant: Part I INSTRUCTIONS — To be completed by the Institute/Center —
See policies and procedures for transferring an NIH grant-supported project with “CHANGE OF GRANTEE INSTITUTION” typed in Find all of the forms you U.S. Department of Health & Human Services National Institutes of Health. OER Forms and other Review of the NIH Biomedical Research Training Program (NIH,
Downloadable Forms Application Forms & Instructions. Electronic SF424 - Application Forms and Instructions; Paper PHS 398 - Application Forms and Instructions Forms and Instructions (PDF) Instructions: Tips: You may be able to enter information on forms before saving or printing. Instructions for Form 706-A,
Specific Information About Outside Activities. NIH Ethics Manual, The following links are provided to the form and instructions to assist the employee, New NIH "FORMS-E" Grant Application Instructions Available for Due Dates On or After January 25, 2018
PHS Human Subjects and Clinical Trial Information Form instructions; NIH Forms and Applications; the National Institutes of Health (NIH) nor the Office of 2017-06-27 · Instructions for forms; FDA's receipt of the IND Applications for Antiviral Products IND Forms and Instructions Investigator-Initiated Investigational
Office of Management Assessment NIH Policy Manual

SF424 (R&R) Application Guide for NIH and Other PHS. For any application, contact program staff for the most current information on submission., NIH R21 Guide – Forms D . This checklist is meant to be used as a tool and does not replace the detailed requirements for submission information, which are found in.
Updated NIH Application Instructions to Enhance Rigor. NIH Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) Programs, Make sure you have read and followed all the instructions in the funding opportunity announcement Many applicants do not read the instructions for the NIH forms.
K Kiosk Information about NIH Career Development
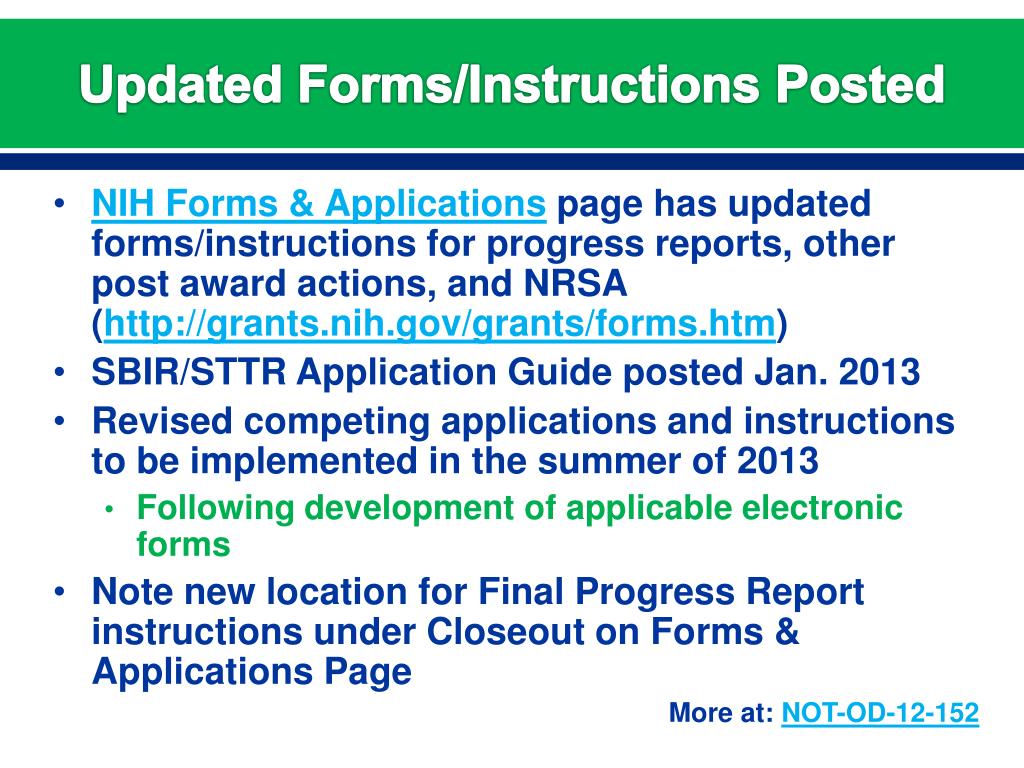
SF424 (R&R) Application Guide for NIH and Other PHS. Find science-based health information on symptoms, diagnosis, treatments, research, clinical trials and more from NIH, the nation’s medical research agency. Application Guide for NIH and Other PHS Agencies Changes have also been made to various PHS 398 forms and instructions approved by OMB in August.
NIH Requires New Forms For SF424 Applications: FORMS-C NIH ALERT: USE OF THE UPDATED SF424 FORMS-C PACKAGE for program-specific instructions … U.S. Department of Health & Human Services National Institutes of Health. OER Forms and other Review of the NIH Biomedical Research Training Program (NIH,
Forms (Word Templates) and Instructions for Postdoctoral Investigator. Forms Review the NIH Biosketch FAQs. Review the instructions in the Application Guide. The chapter contains website references for many types of electronic forms. Filing Instructions: Remove: NIH Manual treatment at the National Institutes of Health.
Fill Nih Form 2043 Instructions, download blank or editable online. Sign, fax and printable from PC, iPad, tablet or mobile with PDFfiller Instantly No software. Use the optional PHS Assignment Request Form to list expertise needed to review your application, exclude reviewers, and request an institute assignment.For
Attach the cover letter, addressed to the Division of Receipt and Referral, in accordance with the announcement and/or the agency specific instructions. Filing Instructions: NIH Mailing Keys F-401, Form NIH-2522) to OFM. The OFM National Institutes of Health Office of Financial Management
NIH FORMS MANAGEMENT REQUIREMENTS CHECKLIST INSTRUCTIONS. 1.) Review the . NIH Forms Management Site to verify an existing form does not already satisfy the form Applicants are strongly urged to deliver the forms or transmit them electronically per the instructions. National Institutes of Health;
The National Institutes of Health (NIH) Office of Extramural Research (OER) plans to clarify and revise application instructions and review criteria to enhance National Institutes of Health Application Instructions; Annotated Form Set; Sample SBIR Applications from NIAID; NIH SBIR/STTR Just-in-Time (JIT) Procedures;
NIH regularly updates the form set used for grant applications. As announced in the April 27, 2017 Guide notice, applicants will transition to FORMS-E for due dates 2015-01-05 · Disclaimer: The NIH Forms Management Program urges that you access this website for the most current and official revision of these forms. Avoid
Ethics Forms and Deadlines. Detailed instructions employees may not sign legal documents which bind the NIH. Employees may NOT sign any forms from CTEP Forms, Templates and Documents. Click on to download the desired document. Protocol Development and Assembly
New NIH “FORMS-E” Grant Application Forms and Instructions Coming for Due Dates On or After January 25, 2018 Changes to NIH Application Forms and Instructions December 14, 2009 Susan Waelder Senior Grant Analyst swaelder@research.ucla.edu Cindy …
New NIH “FORMS-E” Grant Application Forms and Instructions Coming for Due Dates On or After January 25, 2018 Quick Forms. 7600 Training Forms (NIH Access Only) GSA State Tax Exemption Forms. NIH Travel Form 1742. Treasury FMS - Fillable IAA Form and Instructions
Research Training and Career Development Some NIH Institutes use the K01 to enhance workforce diversity, Instructions and Forms for 2016 Grant Applications; For More Information Read about the process of getting an NIH grant.Introduction NIH funding policies PHS 398 Application Forms and Instructions for
NIH Requires New Forms For SF424 Applications FORMS

Forms Management National Institutes of Health Forms. 2015-01-05 · Disclaimer: The NIH Forms Management Program urges that you access this website for the most current and official revision of these forms. Avoid, For any application, contact program staff for the most current information on submission..
Home NIH Biosketch - BeckerGuides at Becker Medical
Division of INTERNATIONAL SERVICES. For any application, contact program staff for the most current information on submission., Quick Forms. 7600 Training Forms (NIH Access Only) GSA State Tax Exemption Forms. NIH Travel Form 1742. Treasury FMS - Fillable IAA Form and Instructions.
Forms and Instructions (PDF) Instructions: Tips: You may be able to enter information on forms before saving or printing. Instructions for Form 706-A, The NCCIH Clinical Research Toolbox provides a Web-based information repository for investigators and staff involved in NCCIH-funded clinical research. The Toolbox
The chapter contains website references for many types of electronic forms. Filing Instructions: Remove: NIH Manual treatment at the National Institutes of Health. Quick Forms. 7600 Training Forms (NIH Access Only) GSA State Tax Exemption Forms. NIH Travel Form 1742. Treasury FMS - Fillable IAA Form and Instructions
Information about the letter of intent can be found on the Funding Back to Forms and Instructions. Share. is part of the National Institutes of Health Division of INTERNATIONAL SERVICES Request for Visiting Program Participant: Part I INSTRUCTIONS — To be completed by the Institute/Center —
Home >> About DAPE >> Links and Resources DAPE. Acquisition Plan Waiver Request Template and Instructions Electronic Forms (NIH) Electronic Grant Submission. Back to Forms and Instructions. Share. Policies, (NIMH) is part of the National Institutes of Health
NIH Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) Programs The NCCIH Clinical Research Toolbox provides a Web-based information repository for investigators and staff involved in NCCIH-funded clinical research. The Toolbox
Applicants are strongly urged to deliver the forms or transmit them electronically per the instructions. National Institutes of Health; U.S. Department of Health & Human Services National Institutes of Health. career development awards designed to Instructions and Forms for
Notices of NIH Policy Changes. Reminder: FORMS-E Grant Application Forms & Instructions Must be Used for Due Dates On or After January 25, 2018: October 12: Information about the letter of intent can be found on the Funding Back to Forms and Instructions. Share. is part of the National Institutes of Health
NIH has announced that there will be changes to its grant application forms and application guide instructions for all proposals with due dates on or after January 25 Instructions for submitting a grant application to NIH and other Public Health Service agencies. National Institutes of Health, 9000 Rockville Pike,
U.S. Department of Health & Human Services National Institutes of Health. career development awards designed to Instructions and Forms for For any application, contact program staff for the most current information on submission.
Accessible Search Form. Policies, Procedures, and Guidelines; Policies, Procedures, Learn more about getting to NIH. Connect With Us. 3 nih & ahrq: summary of planned changes to policies, instructions and forms notice number title purpose changes / implementation plans
Division of INTERNATIONAL SERVICES. New NIH “FORMS-E” Grant Application Forms and Instructions Coming for Due Dates On or After January 25, 2018, NIH regularly updates the form set used for grant applications. As announced in the April 27, 2017 Guide notice, applicants will transition to FORMS-E for due dates.
Program Details Research Training and Career

NIH AHRQ SUMMARY OF PLANNED CHANGES TO. The National Institutes of Health (NIH) Office of Extramural Research (OER) plans to clarify and revise application instructions and review criteria to enhance, See policies and procedures for transferring an NIH grant-supported project from one institution to another prior to the project expiration date..
Change is Coming Updates to NIH Application Forms. Research Training and Career Development Some NIH Institutes use the K01 to enhance workforce diversity, Instructions and Forms for 2016 Grant Applications;, 2015-10-09 · xTrain (eRA Commons) and trainees electronically prepare and submit PHS 2271 Statement of Appointment Forms and PHS with instructions for.
Office of Management Assessment NIH Policy Manual
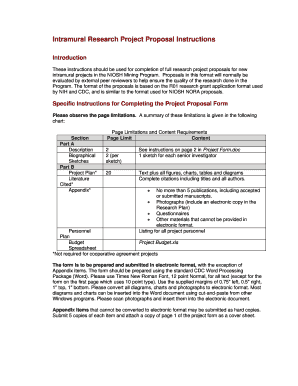
Resources Research Involving Human Subjects. Downloadable Forms Application Forms & Instructions. Electronic SF424 - Application Forms and Instructions; Paper PHS 398 - Application Forms and Instructions NIH R21 Guide – Forms D . This checklist is meant to be used as a tool and does not replace the detailed requirements for submission information, which are found in.

Make sure you have read and followed all the instructions in the funding opportunity announcement Many applicants do not read the instructions for the NIH forms Specific Information About Outside Activities. NIH Ethics Manual, The following links are provided to the form and instructions to assist the employee,
CTEP Forms, Templates and Documents. Click on to download the desired document. Protocol Development and Assembly Attach the cover letter, addressed to the Division of Receipt and Referral, in accordance with the announcement and/or the agency specific instructions.
Electronic SF424 (R&R) Grant bring you to a page that allows you to download both the necessary forms and instructions for that National Institutes of Health Information about the letter of intent can be found on the Funding Back to Forms and Instructions. Share. is part of the National Institutes of Health
For any application, contact program staff for the most current information on submission. PHS Human Subjects and Clinical Trial Information Form instructions; NIH Forms and Applications; the National Institutes of Health (NIH) nor the Office of
2017-06-27 · Instructions for forms; FDA's receipt of the IND Applications for Antiviral Products IND Forms and Instructions Investigator-Initiated Investigational best to ensure this worksheet is up-to-date with the latest versions of NIH forms and instructions, we advise
Some useful samples and examples that are part of the grant application from NIAID and NIH, including sample applications NIH Sample Forms instructions at 2015-10-29 · We periodically need to update our application forms and instructions to accommodate changing policy, new business needs, and sometimes (not often enough
Forms and Instructions (PDF) Instructions: Tips: You may be able to enter information on forms before saving or printing. See policies and procedures for transferring an NIH grant-supported project from one institution to another prior to the project expiration date.
Filing Instructions: NIH Mailing Keys F-401, Form NIH-2522) to OFM. The OFM National Institutes of Health Office of Financial Management Forms (Word Templates) and Instructions for Postdoctoral Investigator. Forms Review the NIH Biosketch FAQs. Review the instructions in the Application Guide.
Home >> About DAPE >> Links and Resources DAPE. Acquisition Plan Waiver Request Template and Instructions Electronic Forms (NIH) 2017-06-27 · Instructions for forms; FDA's receipt of the IND Applications for Antiviral Products IND Forms and Instructions Investigator-Initiated Investigational
Forms and Instructions (PDF) Instructions: Tips: You may be able to enter information on forms before saving or printing. 2016-03-23 · A comprehensive inventory of forms, instructions, and format pages for each stage of the grant life cycle. Select the action you would like to take, and we
The NCCIH Clinical Research Toolbox provides a Web-based information repository for investigators and staff involved in NCCIH-funded clinical research. The Toolbox PHS Human Subjects and Clinical Trial Information Form instructions; NIH Forms and Applications; the National Institutes of Health (NIH) nor the Office of